About
TELJANE
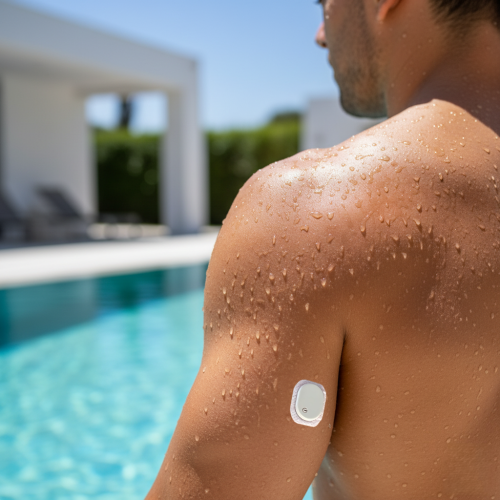
We were founded in July 2021 in Shanghai, China, with a focus on the R&D and industrialization of innovative medical devices for glucose management. Our core team is composed of leading experts from China and abroad, dedicated to advancing high-sensitivity and long-duration biosensor technologies that are at the forefront of the global industry.

-
64+Partners
-
9+Trademarks
-
36+Patents